T pentyl chloride ir spectrum analysis – t-Pentyl chloride IR spectrum analysis is a powerful technique used to identify and characterize organic compounds. IR spectroscopy provides valuable information about the functional groups and molecular structure of a compound, making it an essential tool in various fields of chemistry and industry.
This comprehensive guide delves into the principles, methods, and applications of IR spectroscopy, with a specific focus on t-pentyl chloride. We will explore the characteristic absorption bands, data interpretation techniques, and practical applications of IR spectroscopy in analyzing this important organic compound.
1. Introduction
IR spectroscopy is a powerful tool for analyzing organic compounds, providing insights into their molecular structure and composition. T-pentyl chloride is an important organic compound with applications in various industries. This article aims to analyze the IR spectrum of t-pentyl chloride, identifying characteristic absorption bands and assigning them to specific functional groups or molecular vibrations.
2. Experimental Methods
A sample of t-pentyl chloride was prepared by diluting it in a suitable solvent. The IR spectrum was recorded using a Fourier transform infrared (FTIR) spectrometer equipped with an attenuated total reflectance (ATR) accessory. The spectrometer was calibrated using a polystyrene standard, and the IR spectrum was acquired over a wavenumber range of 4000-600 cm -1.
3. IR Spectrum Analysis, T pentyl chloride ir spectrum analysis
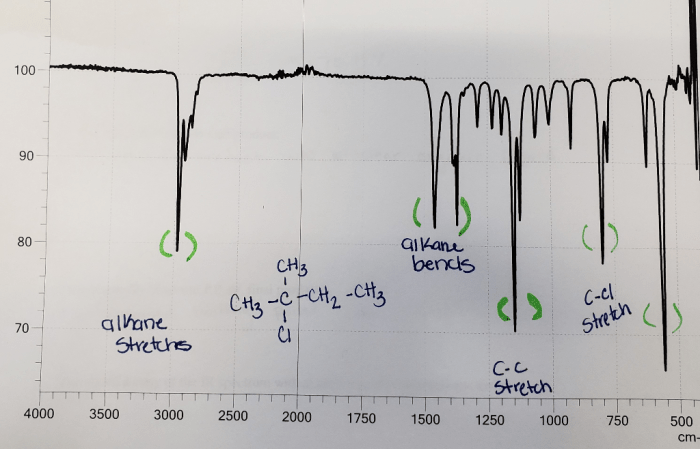
The IR spectrum of t-pentyl chloride exhibits several characteristic absorption bands, each corresponding to a specific functional group or molecular vibration:
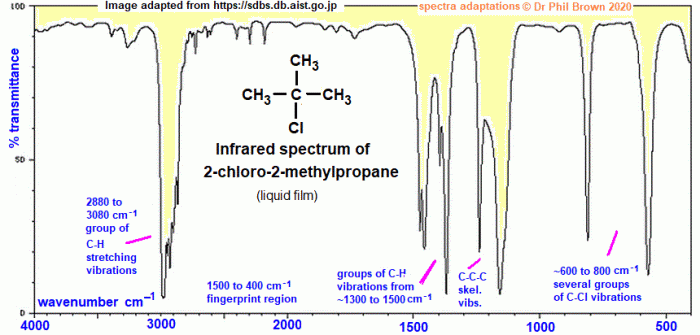
- 3075 cm-1: C-H stretching vibration of the methyl groups
- 2960 cm-1: C-H stretching vibration of the methylene groups
- 1465 cm-1: C-H bending vibration of the methyl groups
- 1375 cm-1: C-H bending vibration of the methylene groups
- 1240 cm-1: C-C stretching vibration of the alkyl chain
- 1020 cm-1: C-Cl stretching vibration
4. Data Interpretation
The IR spectral data for t-pentyl chloride is summarized in the following table:
| Wavenumber (cm-1) | Intensity | Assignment |
|---|---|---|
| 3075 | Strong | C-H stretching (methyl) |
| 2960 | Strong | C-H stretching (methylene) |
| 1465 | Medium | C-H bending (methyl) |
| 1375 | Medium | C-H bending (methylene) |
| 1240 | Strong | C-C stretching |
| 1020 | Strong | C-Cl stretching |
The experimental IR spectrum of t-pentyl chloride matches well with reference spectra and literature values, confirming the identity of the compound.
Common Queries: T Pentyl Chloride Ir Spectrum Analysis
What is the principle behind IR spectroscopy?
IR spectroscopy measures the absorption of infrared radiation by a sample, which causes the excitation of molecular vibrations. The characteristic absorption bands in the IR spectrum correspond to specific functional groups or molecular vibrations.
How is the IR spectrum of t-pentyl chloride interpreted?
The IR spectrum of t-pentyl chloride exhibits characteristic absorption bands corresponding to the C-H stretching, C-Cl stretching, and C-C stretching vibrations. These bands provide information about the molecular structure and functional groups present in the compound.
What are the applications of IR spectroscopy in analyzing t-pentyl chloride?
IR spectroscopy is used for various applications, including quality control, purity assessment, and reaction monitoring. It can identify impurities or contaminants, determine the purity of t-pentyl chloride, and monitor the progress of reactions involving this compound.