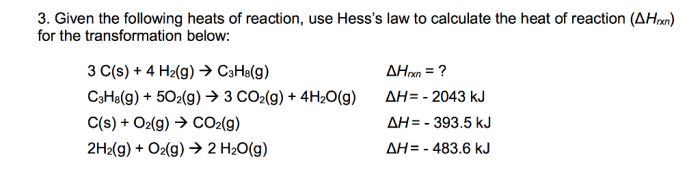
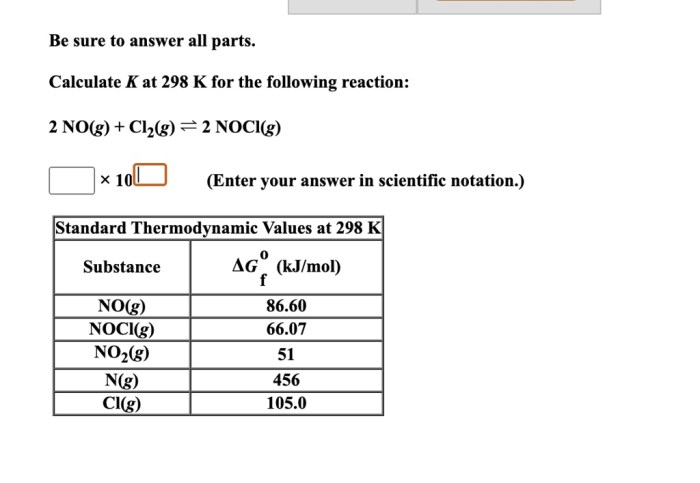
Calculate k at 298 k for the following reaction sets the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail with gaya akademik dengan tone otoritatif and brimming with originality from the outset.
This guide delves into the intricacies of equilibrium constant (K), its temperature dependence, and its applications in various fields, providing a comprehensive understanding of this fundamental concept in chemical reactions.
The significance of K in chemical reactions cannot be overstated. It serves as a quantitative measure of the extent to which a reaction proceeds, allowing us to predict reaction outcomes, design reaction conditions, and understand chemical equilibria. Furthermore, the relationship between K and the Gibbs free energy change (ΔG) provides a powerful tool for calculating K and understanding reaction thermodynamics.
Equilibrium Constant (K)
The equilibrium constant, denoted by K, is a quantitative measure of the extent to which a chemical reaction proceeds towards completion. It is defined as the ratio of the concentrations of the products to the concentrations of the reactants at equilibrium, raised to their respective stoichiometric coefficients.
K provides valuable information about the spontaneity and direction of a reaction. A large K value indicates that the products are favored at equilibrium, while a small K value indicates that the reactants are favored. K also plays a crucial role in predicting the extent of reaction and in designing reaction conditions to achieve desired outcomes.
Significance of K in Chemical Reactions
K is a key parameter in understanding chemical equilibria and reaction behavior. It helps determine the direction and extent of a reaction, providing insights into the relative strengths of reactants and products. A high K value indicates a strong tendency for the reaction to proceed towards completion, while a low K value suggests a limited extent of reaction.
Relationship between K and the Extent of Reaction
The equilibrium constant K is directly related to the extent of reaction, which refers to the fraction of reactants that have been converted into products at equilibrium. A high K value corresponds to a high extent of reaction, indicating that a significant portion of the reactants have been consumed to form products.
Conversely, a low K value implies a low extent of reaction, suggesting that only a small fraction of the reactants have been converted.
Temperature Dependence of K
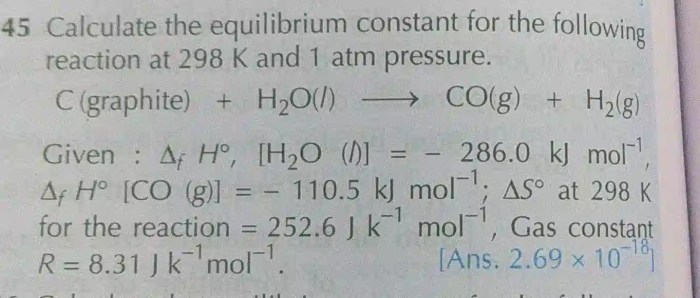
The equilibrium constant K is temperature-dependent. The effect of temperature on K is described by the van’t Hoff equation:
ln(K2/K 1) = -ΔH°/R(1/T 2– 1/T 1)
where:
- K 1and K 2are the equilibrium constants at temperatures T 1and T 2, respectively
- ΔH° is the standard enthalpy change of the reaction
- R is the ideal gas constant (8.314 J/mol·K)
The van’t Hoff equation shows that the equilibrium constant K increases with increasing temperature for exothermic reactions (ΔH° < 0) and decreases with increasing temperature for endothermic reactions (ΔH° > 0).
Examples of How Temperature Affects K in Specific Reactions
The temperature dependence of K can be observed in various chemical reactions. For instance, in the exothermic reaction of hydrogen and iodine to form hydrogen iodide:
H2+ I 2⇌ 2HI
The equilibrium constant K increases with increasing temperature, indicating that the formation of hydrogen iodide is favored at higher temperatures.
Reaction Thermodynamics
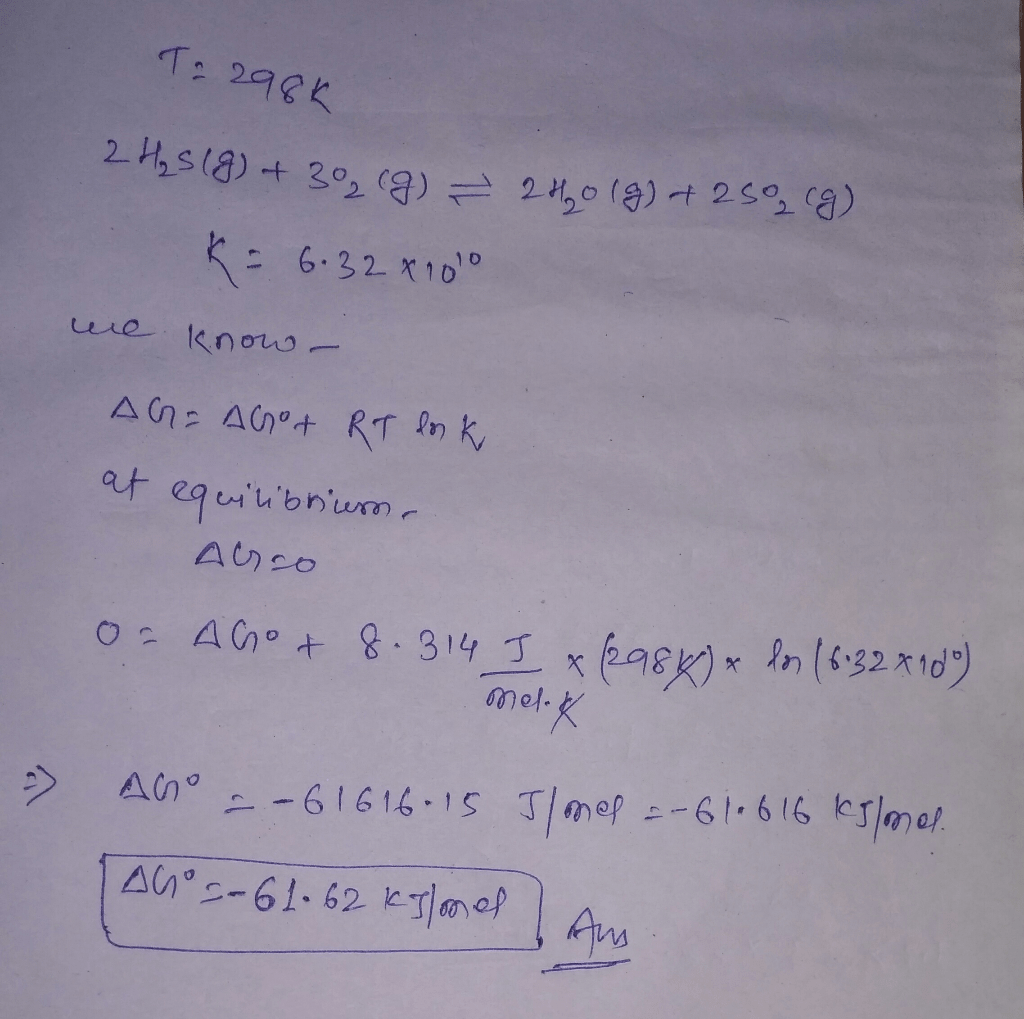
The equilibrium constant K is closely related to the Gibbs free energy change (ΔG) of the reaction. ΔG is a measure of the spontaneity of a reaction and is defined as:
ΔG =-RTlnK
where:
- R is the ideal gas constant (8.314 J/mol·K)
- T is the temperature in Kelvin
- K is the equilibrium constant
The Gibbs free energy change can be used to calculate the equilibrium constant K. A negative ΔG indicates that the reaction is spontaneous and proceeds towards completion, while a positive ΔG indicates that the reaction is non-spontaneous and requires an input of energy.
Examples of Calculating ΔG and K for Different Reactions
The relationship between ΔG and K can be used to calculate both quantities for different reactions. For example, in the reaction:
A + B ⇌ C + D
If the ΔG° of the reaction is -10 kJ/mol at 298 K, the equilibrium constant K can be calculated using the equation:
ΔG° =-RTlnK
Substituting the values into the equation gives:
-10 kJ/mol =-(8.314 J/mol·K)(298 K)lnK
Solving for K gives:
K = 1.0 x 103
Applications of K
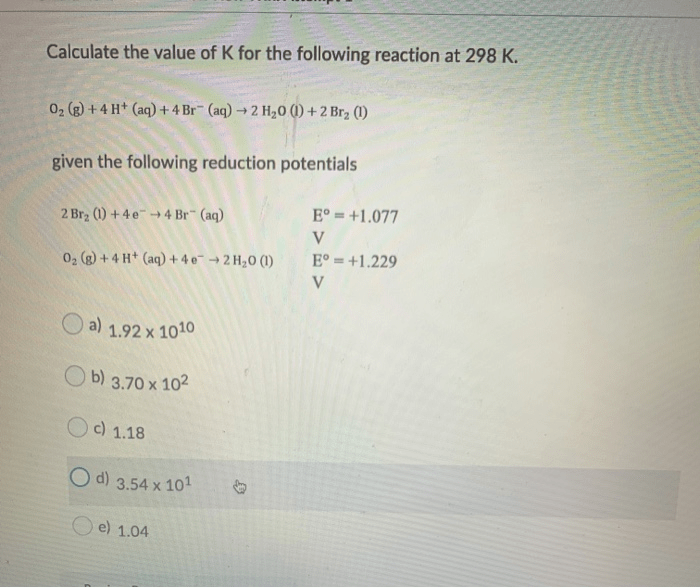
The equilibrium constant K has numerous practical applications in various fields, including:
Predicting Reaction Outcomes, Calculate k at 298 k for the following reaction
K can be used to predict the outcome of a reaction by comparing its value to 1. If K > 1, the reaction will proceed towards completion, forming more products than reactants. If K< 1, the reaction will favor the formation of reactants over products.
Designing Reaction Conditions
K can be used to design reaction conditions that optimize the yield of desired products. By manipulating temperature, pressure, or concentration, K can be adjusted to shift the equilibrium towards the desired direction.
Understanding Chemical Equilibria
K provides insights into the equilibrium behavior of chemical reactions. It helps determine the relative amounts of reactants and products at equilibrium and allows for the prediction of reaction outcomes under different conditions.
Popular Questions: Calculate K At 298 K For The Following Reaction
What is the significance of K in chemical reactions?
K is a quantitative measure of the extent to which a reaction proceeds, allowing us to predict reaction outcomes, design reaction conditions, and understand chemical equilibria.
How does temperature affect K?
Temperature has a significant effect on K, as described by the van’t Hoff equation. In general, increasing temperature increases K for exothermic reactions and decreases K for endothermic reactions.
What is the relationship between K and ΔG?
K and ΔG are related by the equation ΔG = -RTlnK, where R is the gas constant and T is the temperature. This relationship allows us to calculate K from ΔG and vice versa.