From your observations of the chemical and physical properties of substances, a captivating journey of discovery unfolds, where the secrets of matter are laid bare. This exploration delves into the intricate characteristics that define substances, empowering us to identify, characterize, and understand the world around us.
Chemical reactions, physical changes, and the application of spectroscopic and microscopic techniques provide invaluable tools for unraveling the mysteries of matter. Each method offers unique insights, revealing the dynamic nature of substances and their interactions.
Chemical and Physical Observations
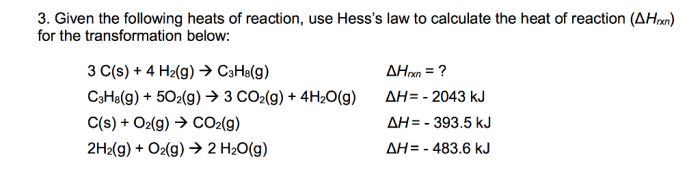
Chemical and physical observations are important tools for identifying and characterizing substances. Chemical observations involve observing the chemical properties of a substance, such as its reactivity, flammability, and solubility. Physical observations involve observing the physical properties of a substance, such as its color, shape, and density.
Chemical observations can be used to identify a substance by comparing its reactivity to known substances. For example, a substance that reacts with an acid to produce a gas is likely to be a carbonate. Physical observations can also be used to identify a substance by comparing its properties to known substances.
For example, a substance that is white, crystalline, and soluble in water is likely to be sugar.
Limitations of Using Chemical and Physical Observations for Identification Purposes, From your observations of the chemical and physical
- Chemical and physical observations can only be used to identify substances that have been previously characterized.
- Chemical and physical observations can be subjective, and different observers may interpret the same observations differently.
- Chemical and physical observations can be affected by the presence of impurities.
Chemical Reactions

Chemical reactions are processes in which atoms or molecules rearrange themselves to form new substances. Chemical reactions can be used to identify and characterize substances by observing the products of the reaction. For example, a substance that reacts with an acid to produce a gas is likely to be a carbonate.
Chemical reactions can also be used to characterize substances by observing the changes in their physical properties. For example, a substance that turns red when it is heated is likely to be an indicator.
Limitations of Using Chemical Reactions for Identification Purposes
- Chemical reactions can only be used to identify substances that react with the reagents that are used.
- Chemical reactions can be dangerous, and it is important to take precautions when performing chemical reactions.
- Chemical reactions can be time-consuming, and it may not be possible to identify a substance quickly using chemical reactions.
Physical Changes
Physical changes are changes in the physical properties of a substance without changing its chemical composition. Physical changes can be used to identify and characterize substances by observing the changes in their physical properties. For example, a substance that melts when it is heated is likely to be a solid.
Physical changes can also be used to characterize substances by observing the changes in their reactivity. For example, a substance that becomes more reactive when it is heated is likely to be a reactant.
Limitations of Using Physical Changes for Identification Purposes
- Physical changes can only be used to identify substances that undergo physical changes.
- Physical changes can be subjective, and different observers may interpret the same observations differently.
- Physical changes can be affected by the presence of impurities.
Spectroscopic Techniques: From Your Observations Of The Chemical And Physical
Spectroscopic techniques are methods for analyzing substances by measuring the interaction of electromagnetic radiation with the substance. Spectroscopic techniques can be used to identify and characterize substances by observing the absorption, emission, or scattering of electromagnetic radiation.
Spectroscopic techniques can be used to identify a substance by comparing its spectrum to the spectra of known substances. Spectroscopic techniques can also be used to characterize a substance by observing the changes in its spectrum under different conditions.
Limitations of Using Spectroscopic Techniques for Identification Purposes
- Spectroscopic techniques can only be used to identify substances that have been previously characterized.
- Spectroscopic techniques can be expensive and time-consuming.
- Spectroscopic techniques can be affected by the presence of impurities.
Microscopic Techniques
Microscopic techniques are methods for analyzing substances by observing them under a microscope. Microscopic techniques can be used to identify and characterize substances by observing their size, shape, and structure.
Microscopic techniques can be used to identify a substance by comparing its appearance to the appearance of known substances. Microscopic techniques can also be used to characterize a substance by observing the changes in its appearance under different conditions.
Limitations of Using Microscopic Techniques for Identification Purposes
- Microscopic techniques can only be used to identify substances that are large enough to be seen under a microscope.
- Microscopic techniques can be subjective, and different observers may interpret the same observations differently.
- Microscopic techniques can be affected by the presence of impurities.
Expert Answers
What are the limitations of using chemical reactions for identification purposes?
Chemical reactions can be limited by factors such as the availability of reactants, reaction rates, and the potential for side reactions. Additionally, some substances may not undergo specific reactions, limiting their usefulness for identification.
How do spectroscopic techniques aid in substance identification?
Spectroscopic techniques analyze the interaction of electromagnetic radiation with substances, providing information about their molecular structure and composition. This data can be used to identify and differentiate substances based on their unique spectral signatures.